According to a recent report by an agency, due to stricter emission regulations, lower cost of electric vehicle technology, and increased consumer willingness to purchase, in the developed affluent cities of London and Singapore in 2030, there will be three points in the cars on the road. The second is electric vehicles. Electric vehicles are now very common. In order to reduce harmful greenhouse gas emissions, governments have promoted the development of the electric vehicle market by reducing taxes and fees, providing subsidies and setting up low-emission areas. Electric car sales and battery cost decline The technical cost of electric vehicles is also rapidly decreasing. In 2015, the cost of lithium batteries was reduced by as much as 65%, the cost per kilowatt hour was only $350, and in 2010 it was as high as $1,000 per kilowatt hour. . In the next decade, the cost of electric vehicle batteries is expected to be further reduced to less than $100 per kWh. According to the report, in high-density and high-income cities such as London and Singapore, driven by factors such as restricted areas, consumer willingness and economic stimulus, electric vehicles will account for 60% of road vehicles in 2030. The rise of electric vehicles will also threaten the traditional automotive manufacturing industry. The automotive industry is facing a challenge that is completely different from the future of the past, and the transition from a pure product ownership model to the provision of transportation services. Fuel retailers also need to start thinking about further realizing existing assets while trying to get more out of the electric vehicle business, such as charging services, retail markets and fleet services. The report pointed out that the intertwining of various trends, including the decentralization of energy and the popularity of the Internet of Things, will cause major changes in the transportation system in the next 10-15 years.
Stainless steel does not readily corrode, rust or stain with water as ordinary steel does. However, it is not fully stain-proof in low-oxygen, high-salinity, or poor air-circulation environments.There are different grades and surface finishes of stainless steel to suit the environment the alloy must endure. Stainless steel is used where both the properties of steel and corrosion resistance are required.
Products Detail:
Machining
MAIN PARTICULARS
Material
Aluminum, Stainless steel, Carbon steel, Alloy steel, Brass, Casting iron, Bronze, Powder metal, Plastic
Standard
ASTM, ASME, DIN, JIS, ISO, BS, API, EN
Certificate
ISO9001, BV
Processing
CNC turning, CNC turning and milling compound processing, 3/4/5 axis CNC milling, wire-cutting, EDM, grinding etc.
Finishing surface
Machined surface with tectyl891, anodize, polishing, nickel plating, zinc plating and chrome plating, oxidation, powder coating, painting, etc.
Inspection
material, construction, dimension, heat treatment, hardness, NDT
Quality
ISO9001, BV, control production
Stainless steel differs from carbon steel by the amount of chromium present. Unprotected carbon steel rusts readily when exposed to air and moisture. This iron oxide film (the rust) is active and accelerates corrosion by forming more iron oxide; and, because of the greater volume of the iron oxide, this tends to flake and fall away. Stainless steels contain sufficient chromium to form a passive film of chromium oxide, which prevents further surface corrosion by blocking oxygen diffusion to the steel surface and blocks corrosion from spreading into the metal's internal structure.Passivation occurs only if the proportion of chromium is high enough and oxygen is present.
Stainless property
Acids Cnc Stainless Steel Parts,Oem Cnc Stainless Steel Parts,Custom Stainless Steel Parts,Cnc Stainless Steel Turning Parts Shinvast Industry Ltd , http://www.shinvastindustry.com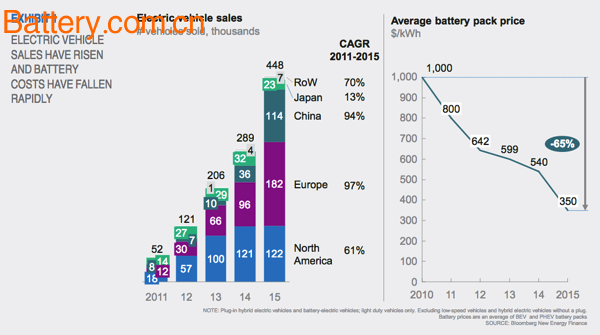
Oxidation
High oxidation resistance in air at ambient temperature is normally achieved with additions of a minimum of 13% (by weight) chromium, and up to 26% is used for harsh environments.[4] The chromium forms a passivation layer of chromium(III) oxide (Cr2O3) when exposed to oxygen. The layer is too thin to be visible, and the metal remains lustrous and smooth. The layer is impervious to water and air, protecting the metal beneath, and this layer quickly reforms when the surface is scratched. This phenomenon is called passivation and is seen in other metals, such as aluminium and titanium. Corrosion resistance can be adversely affected if the component is used in a non-oxygenated environment, a typical example being underwater keel bolts buried in timber.
When stainless steel parts such as nuts and bolts are forced together, the oxide layer can be scraped off, allowing the parts to weld together. When forcibly disassembled, the welded material may be torn and pitted, an effect known as galling. This destructive galling can be avoided by the use of dissimilar materials for the parts forced together, for example bronze and stainless steel, or even different types of stainless steels (martensitic against austenitic). However, two different alloys electrically connected in a humid environment may act as a voltaic pile and corrode faster. Nitronic alloys made by selective alloying with manganese and nitrogen may have a reduced tendency to gall. Additionally, threaded joints may be lubricated to prevent galling. Low-temperature carburizing is another option that virtually eliminates galling and allows the use of similar materials without the risk of corrosion and the need for lubrication.
Stainless steel is generally highly resistant to attack from acids, but this quality depends on the kind and concentration of the acid, the surrounding temperature, and the type of steel. Type 904 is resistant to sulfuric acid at room temperature, even in high concentrations; type 316 and 317 are resistant below 10%, and 304 should not be used in the presence of sulfuric acid at any concentration. All types of stainless steel resist attack from phosphoric acid, 316 and 317 more so than 304; types 304L and 430 have been successfully used with nitric acid. Hydrochloric acid will damage any kind of stainless steel, and should be avoided.
October 15, 2019